Publication Spotlight: The C99 domain of the amyloid precursor protein resides in the disordered membrane phase.
Jun 21st 2022
Capone R, Tiwari A, Hadziselimovic A, Peskova Y, Hutchison JM, Sanders CR, Kenworthy AK. The C99 domain of the amyloid precursor protein resides in the disordered membrane phase. J Biol Chem. 2021 Jan-Jun;296:100652. doi: 10.1016/j.jbc.2021.100652. Epub 2021 Apr 9. PMID: 33839158; PMCID: PMC8113881.
The C99 domain of the amyloid precursor protein resides in the disordered membrane phase.
Abstract
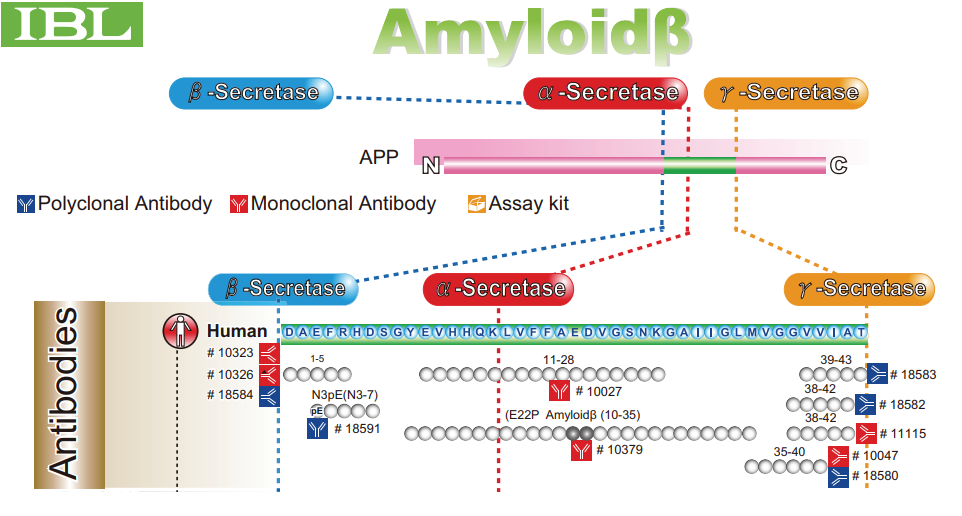
Processing of the amyloid precursor protein (APP) via the amyloidogenic pathway is associated with the etiology of Alzheimer's disease. The cleavage of APP by β-secretase to generate the transmembrane 99-residue C-terminal fragment (C99) and subsequent processing of C99 by γ-secretase to yield amyloid-β (Aβ) peptides are essential steps in this pathway. Biochemical evidence suggests that amyloidogenic processing of C99 occurs in cholesterol- and sphingolipid-enriched liquid-ordered phase membrane rafts. However, direct evidence that C99 preferentially associates with these rafts has remained elusive. Here, we tested this by quantifying the affinity of C99-GFP for raft domains in cell-derived giant plasma membrane vesicles (GPMVs). We found that C99 was essentially excluded from ordered domains in vesicles from HeLa cells, undifferentiated SH-SY5Y cells, or SH-SY5Y-derived neurons; instead, ∼90% of C99 partitioned into disordered domains. The strong association of C99 with disordered domains occurred independently of its cholesterol-binding activity or homodimerization, or of the presence of the familial Alzheimer disease Arctic mutation (APP E693G). Finally, through biochemical studies we confirmed previous results, which showed that C99 is processed in the plasma membrane by α-secretase, in addition to the well-known γ-secretase. These findings suggest that C99 itself lacks an intrinsic affinity for raft domains, implying that either i) amyloidogenic processing of the protein occurs in disordered regions of the membrane, ii) processing involves a marginal subpopulation of C99 found in rafts, or iii) as-yet-unidentified protein-protein interactions with C99 in living cells drive this protein into membrane rafts to promote its cleavage therein.
In the study the IBL Amyloid Beta (N) (82E1) AΒ Anti-Human Mouse IgG MoAb was used to detect the N-terminus of C99/Aβ, blots were probed with mouse IgG mAb 82E1 (IBL cat# 10323; lot 1D-421) used at 0.5 μg/ml.
Learn more about Amyloid Beta (N) (82E1) AΒ Anti-Human Mouse IgG MoAb as well as other publications using the unique antibody.