Publication Spotlight: A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure.
Jan 26th 2023

The IBL-Ameirca Radioimmunoassay (RIA) for Angiotensin (ANG) II was used in this study.
D. Clark Files, Kevin W. Gibbs, Christopher L. Schaich, Sean P. Collins, TanYa M. Gwathmey, Jonathan D. Casey, Wesley H. Self, and Mark C. Chappell 07 JUL 2021 https://doi.org/10.1152/ajplung.00129.2021
A pilot study to access the circulating renin-angiotensin system in COVID-19 acute respiratory failure.
Abstract
The renin-angiotensin system (RAS) is fundamental to COVID-19 pathobiology, due to the interaction between the SARS-CoV-2 virus and the angiotensin-converting enzyme 2 (ACE2) coreceptor for cellular entry. The prevailing hypothesis is that SARS-CoV-2-ACE2 interactions lead to an imbalance of the RAS, favoring proinflammatory angiotensin II (ANG II)-related signaling at the expense of the anti-inflammatory ANG-(1–7)-mediated alternative pathway. Indeed, multiple clinical trials targeting this pathway in COVID-19 are underway. Therefore, precise measurement of circulating RAS components is critical to understand the interplay of the RAS on COVID-19 outcomes. Multiple challenges exist in measuring the RAS in COVID-19, including improper patient controls, ex vivo degradation and low concentrations of angiotensins, and unvalidated laboratory assays. Here, we conducted a prospective pilot study to enroll 33 patients with moderate and severe COVID-19 and physiologically matched COVID-19-negative controls to quantify the circulating RAS. Our enrollment strategy led to physiological matching of COVID-19-negative and COVID-19-positive moderate hypoxic respiratory failure cohorts, in contrast to the severe COVID-19 cohort, which had increased severity of illness, prolonged intensive care unit (ICU) stay, and increased mortality. Circulating ANG II and ANG-(1–7) levels were measured in the low picomolar (pM) range. We found no significant differences in circulating RAS peptides or peptidases between these three cohorts. The combined moderate and severe COVID-19-positive cohorts demonstrated a mild reduction in ACE activity compared with COVID-19-negative controls (2.2 ± 0.9 × 105 vs. 2.9 ± 0.8 × 105 RFU/mL, P = 0.03). These methods may be useful in designing larger studies to physiologically match patients and quantify the RAS in COVID-19 RAS augmenting clinical trials.
Background
Binding of SARS-CoV-2 to the angiotensin-converting enzyme 2 (ACE2) coreceptor for viral entry has focused attention on the renin-angiotensin system (RAS) in the pathophysiology of COVID-19. The prevailing hypothesis is that SARS-CoV-2-ACE2 binding/internalization increases the ratio of angiotensin II (ANG II) to ANG-(1–7) due to loss of ACE2 activity, promoting oxidative stress, inflammation, fibrosis, and increased vascular tone (1, 2). This finding has prompted speculation on the role of the RAS as a disease modifier, and whether pharmacological manipulation of the RAS might improve outcomes in patients with COVID-19...
Methods
The study included patients ≥18 yr of age; with symptoms of acute respiratory infection, with one or more of the following symptoms: cough, fever (>37.5°C/99.5°F), shortness of breath, or sore throat; and tested for SARS-CoV-2 by PCR within the last 10 days with planned admission to the study hospital...
Results
The COVID-19-negative and COVID-19-positive moderate AHRF cohorts were physiologically similar, with a modified sequential organ failure assessment (mSOFA) score of 1.3 ± 0.5 vs. 1.8 ± 0.7 (P = 0.5) and a fraction of inspired oxygen (
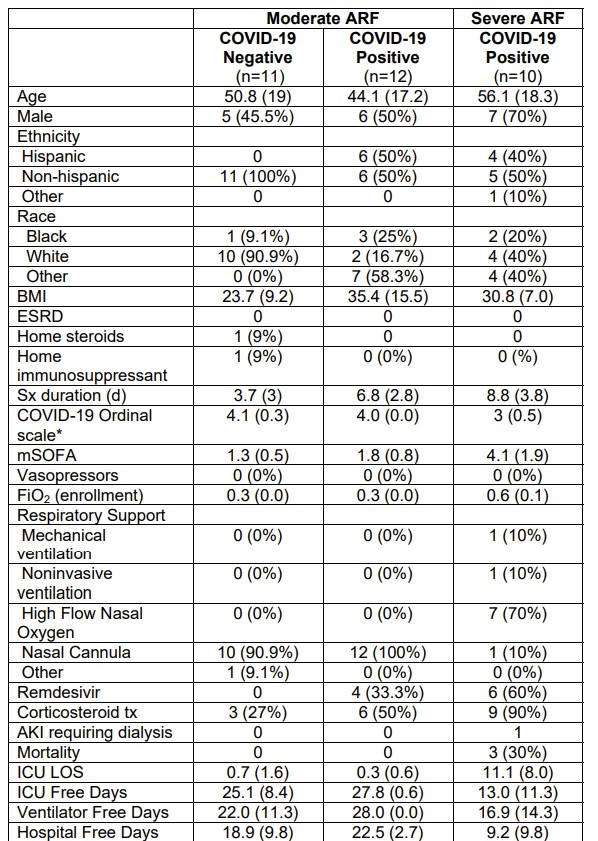
Median [interquartile range (IQR)] plasma ANG-(1–7) and ANG II values were 10.8 [7.1,16.2] and 15.3 [8.0,31.1] pM, respectively, and did not differ by the three study groups (Fig. 1). Three patients (13.6%) in the COVID-19-positive cohorts had ANG-(1–7) values greater than one standard deviation above the mean (107 to 217 pM range), whereas the maximum ANG-(1–7) concentration in the COVID-19-negative cohort was 19 pM. Serum ACE, ACE2, and POP activities were also similar in the study groups (Fig. 1).
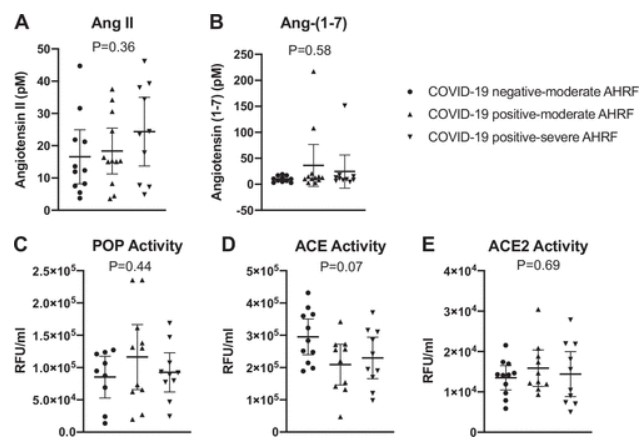
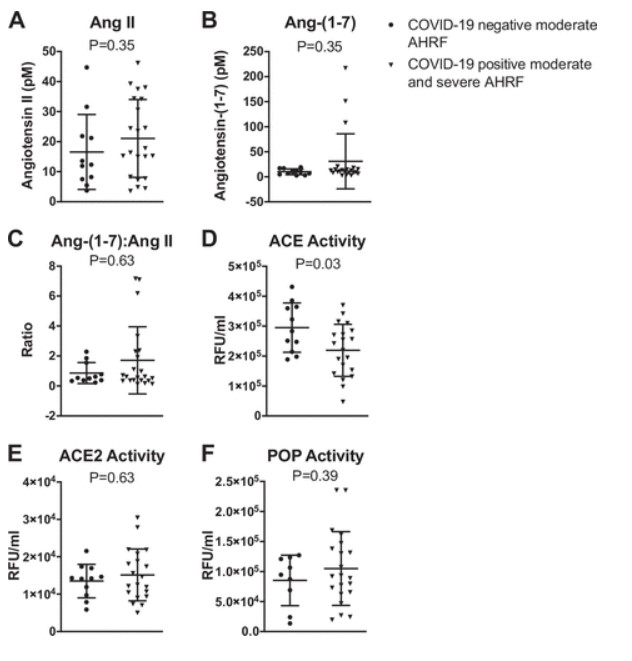
We further analyzed the data by grouping the moderate and severe COVID-19-positive cohorts and comparing them with COVID-19-negative patients. Using these criteria, we also found no differences in ANG II, ANG-(1–7), ACE2, or POP in COVID-19-negative versus COVID-19-positive patients. We did find that ACE activity was reduced in the COVID-19-positive patients versus COVID-19-negative patients (2.2 ± 0.9 × 105 vs. 2.9 ± 0.8 × 105 RFU/mL, P = 0.03) (Fig. 2). The ANG-(1–7):ANG II ratios that are often reported as surrogates for ACE2 activity, did not correlate with either ACE2 or POP activities (Fig. 3).
Discussion
In this prospective pilot study, we enrolled patients with moderate and severe COVID-19 and COVID-19-negative physiologically matched controls. We measured five components of the circulating RAS with validated measures and obtained values that are well within the physiological concentrations accepted in the field (6). This information may be useful for future studies quantifying the circulating RAS in COVID-19 and the effect of RAS augmentation on circulating angiotensins...
The IBL-Ameirca Radioimmunoassay (RIA) for Angiotensin (ANG) II was used in this study.

