The Revival of Peptide Therapeutics
Apr 23rd 2020
All life on Earth is driven by a cascade of complex
and coordinated chemical interactions involving a plethora of proteins at the
molecular level. Specific protein-protein interactions (PPIs) lays the
foundation for all cellular processes that directly or indirectly
regulate a series of enzymatic activities from ion transportation,
transcription of nucleic acids and various post-translational modifications of
translated proteins. Several protein interaction networks have been
delineated that are involved in pathogenesis and disease progression. Tweaking
disease associated PPIs remains the most widely used therapeutic strategy in
multiple diseases including cancers, diabetes, cardiovascular diseases,
infections and other metabolic disorders.
Categorizing therapeutic agents
The two main classes of therapeutic agents used in the clinic are small molecules and biologics. Small molecules comprise of chemical molecules (Mw < 500 Da), which is the most common form of drugs. They are usually taken orally and show rapid effects. Biologics represent mostly protein based drugs like antibodies (Mw > 5000 Da). Antibodies are usually injected into the blood stream and exert very specific and long-lasting effects. When it comes to targeting intracellular PPIs, both these classes of drugs have their limitations. Small molecules can easily cross the membrane barrier but is ineffective in cases of mutations and large interface surfaces involved in PPIs. Antibodies on the other hand, have been successfully used to inhibit cell surface proteins but fail to act on intracellular pathways. A new class of pharmaceutical compounds that functionally fall in between the small molecules and biologics are peptides.
Peptides are short amino acid chains of <40 aa. Like small molecules they can be manufactured easily and exert their effects instantaneously. Also, like biologics they comprise of amino acids and act on very specific targets. Combined with their better invasive ability, adequate binding affinity, lower toxicity and target specificity, peptides make for a very promising class of drug candidates to target intracellular PPIs. Currently, there has been a lot of development in high throughput screening methods and computation-aided rational drug designing that help to identify precise bioactive peptides. Metabolomic, proteomic, and genomic screening of toxins and other natural compounds can lead to the discovery of potential therapeutic peptides that may contain unique structural features generated by uncommon post-translational modifications or non-ribosomal synthesis.
History of peptide therapeutics
The use of peptides for therapy began as early as the 1920s. Initially peptides were used as “replacement therapies” or supplements in the absence of endogenous production. The first peptide hormone therapy was the administration of insulin (isolated from animal tissues) for diabetic patients. This was followed by the isolation of other hormones like ACTH and calcitonin from natural sources for treatment. The advancements in the genomic era saw molecular characterization of proteins and peptides, enabling production of synthetic and recombinant enzymes or hormones.
However, certain limitations with peptide drugs like short plasma half-life and negligible oral bioavailability stalled the progression of peptide therapeutics in the later half of the 20th century. One of the main challenges faced was drug inactivation by endogenous peptidases and speedy excretory clearance. Another leap in this area of therapeutics happened in 1980s when Krenning et al used radiolabelled peptide analog of somatostatin (SST) to target neuroendocrine tumors. The concept of using peptides as a targeting moiety for cancer diagnosis and treatment, instead of an antibody, kick-started further developments. Currently more than 500 peptides have been approved for therapy in the United States, Europe and Japan. The number of peptides entering clinical have been gradually increasing ever since.
Improving pharmaceutical properties
Peptides are susceptible to degradation by peptidases and have a very short half-life. Moreover, they can be easily broken down into amino acids by the enzymes in the GI tract making them unsuitable for oral prescription. Numerous strategies have been applied to enhance the pharmaceutical properties of peptide drugs, including stability, solubility, bioavailability and permeability.
- Peptide conjugates - Conjugating the peptides to various moieties like polyethylene glycol (PEG), lipids, and Fc fragments has been used to increase half-life and extend renal clearance time. Conjugation can also be used to deliver a cytotoxic or imaging agent (for diagnostic use) to specific cell types targeted by the peptide.
- Conformational modifications - Incorporating cyclic structures, intramolecular di-sulphide bonds, D-amino acids or modifying the C-and N- terminus have shown to improve the stability making them less prone to protease degradation and more permeable through the gut membrane. This method is often referred to as peptidomimetics, wherein the analogs exert the same biological effects but are more stable in-vivo.
- Alternative delivery methods - New adjuvant and carrier systems have been developed to improve the bioavailability of peptide drugs. Microemulsions, nanoparticles and liposomes are some of the novel strategies being considered for oral prescription along with the use of penetration enhancers or enzymatic inhibitors (Preet P., 2018). Another new delivery system using mucoadhesive polymers have shown increased permeability of the drug candidates across epithelial membranes while inhibiting peptidolytic enzymes.
Oral prescriptions have better patient compliance and hence there has been a lot of effort to improve the oral bioavailability of peptide drugs. There has been some success with chemical modification methods which can enhance both peptide drug enzymatic stability and permeability in vivo.
Peptides used in different therapeutic areas
For many years peptide therapeutics meant replacement therapy for natural peptides or hormones. With advancements in peptide chemistry and computational modelling, therapeutic grade peptides can be designed to target PPIs in multiple disease areas. Peptides can be used as a delivery vehicle for diagnostic agents and/or drugs. Peptide drugs are mainly function as agonists or antagonists to disease associated signalling pathways. Peptides can prove advantageous over antibodies due to their non-immunogenicity, fast blood clearance, higher tissue diffusion owing to the lower molecular weight and better tolerability by patients.
Cancer therapy
Peptides can be employed in numerous ways to target cancer (Figure 1). One of the major strategies is the use of inhibitory or interference peptides (iPeps) designed to block specific proteins involved in cancer progression. Peptides can also be explored as tumor targeting agents that carry cytotoxic drugs and radionuclides or diagnostic tools and vaccines.
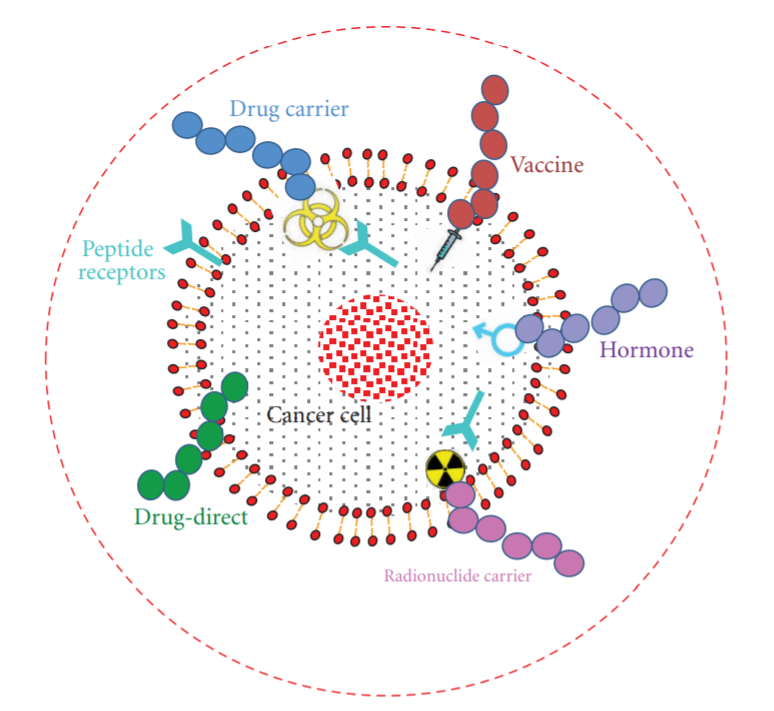
Figure 1: Different possible treatment options of cancer using peptides. Peptides can be used as anticancer drug, cytotoxic drug carrier, vaccine, hormones, and radionuclide carrier. (J. Thundimadathil, 2012)
- iPeps - Interference peptides are being developed against several oncoproteins and oncogenic transcription factors. Some of the targets currently in pre-clinical and clinical phases include MYC, HOX, KRAS, BCL2, HDM2 (Sorolla A. et al., 2020). Since iPeps target intracellular proteins, it is important to make sure that the peptides are internalised and remain stable. Hence, conjugation of cell penetrating peptides and nuclear localization sequences might be necessary. It is also essential to increase the in-vivo stability by methods like modification of N-C-termini, D-amino acids or cyclization. Moreover, PEGylation can be used to prevent immunogenicity.
- Cytotoxic drug carriers - Another strategy to ‘seek and kill’ cancer cells is to conjugate peptides with radionuclides or cytotoxic compounds. Peptide Receptor Radionuclide Therapy (PRRT) via radiolabeled somatostatin analogs (Figure 2) is the gold standard in treating neuroendocrine tumors (NETs) (Thundimadathil J., 2012). The radiolabels usually used are Indium-111, Yttrium-90 or Lutetium-177. Likewise, cytotoxic compounds linked to analogs of hormonal peptides like LHRH (luteinizing hormonereleasing hormone), bombesin, and somatostatin are used to target specific tumor types that possess receptors for these facilitating internalization and selective killing of cancer cells.
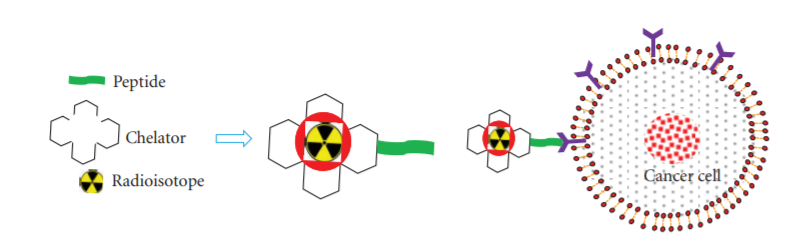
Figure 2: Peptide receptor radionuclide therapy (PRRT); radiolabeled somatostatin analogs generally comprise three main parts: a cyclic octapeptide (e.g., Tyr3-octreotide or Tyr3-octreotate), a chelator (e.g., DTPA or DOTA), and a radioactive element. Radioisotopes commonly used in PRRT are 111In, 90Y, and 177Lu. (J. Thundimadathil, 2012)
- Peptide cancer vaccines - The concept of cancer vaccines stems from the identification of tumor-associated antigens (TAAs) expressed on tumor cells. These TAA peptides mixed with an adjuvant can be used to activate the host immune system de-novo or to heighten the anti-tumor response. Several peptide vaccines have demonstrated promising results in clinical trials. Some of the notable peptide vaccines are HER-2/neu immunodominant peptide (for lung, breast, or ovarian cancer), Mucin-1 peptide (MUC-1, Stimuvax) for breast or colon cancer, Carcinoembryonic antigen for colorectal, gastric, breast, pancreatic and non-small-cell lung cancers, and HPV-16 E7 peptide for cervical cancer.
- Type 2 Diabetes - Peptides as therapy for diabetes started as insulin replacement therapies in the 1920s. Apart from insulin, several other hormones involved in the incertin effect (insulin secretory response to oral glucose) have been developed for treating type 2 diabetes (Figure 3).
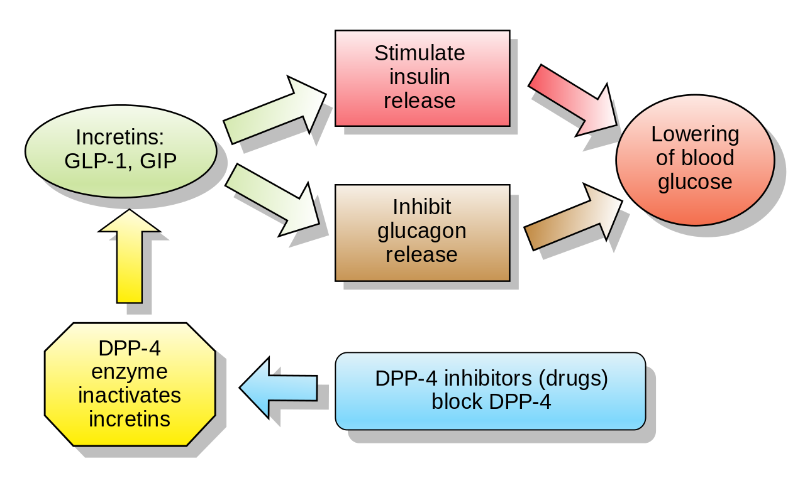
Figure 3: The effect of incertin hormones, GLP-1 (Glucagon-like peptide-1) and GIP (Glucose-dependant insulinotropic hormone), DPP-4 (Dipeptidyl peptidase-4) (www.wikipedia.com)
- GLP-1 receptor agonists - GLP-1 and GIP are peptide hormones that get activated in the gut during ingestion of food. In many type 2 diabetic patients, the GLP-1 hormone levels have been reported to be low but the insulinotropic response is maintained. To the contrary, most patients show normal GIP levels but low responsiveness to GIP hormone. This makes GLP-1 a favourable therapeutic target but natural GLP-1 being easily metabolised and having a very short half-life, makes it difficult to be used in the clinic. Several analogues have been developed like exendin-4 and albumin-bound GLP-1 derivatives that are resistant to degradation and therefore extend circulation time.
- DPP-IV inhibitors - DPP-IV enzyme degrades GLP-1, thereby inactivating it. The inhibition of DPP-IV is an alternative approach to keep the incertin effect high. Multiple DPP-IV inhibitors have been characterized and are available in the market to lower blood glucose by prolongation of GLP-1 and GIP action.
Cardiovascular diseases
Prevention of cardiovascular diseases usually involves regulation of causative risk factors such as hypercholesterolemia, inflammation, hyperglycaemia, obesity, insulin resistance or high blood pressure. Lately, peptide drugs are being designed to intervene pathogenesis at the protein signalling level. Some of the targets under development are listed below.
- ApoA-I is known to play a critical role in cholesterol transport and cellular cholesterol homeostasis. Several animal studies have demonstrated the protective effects of apoA-I in inflammation, vascular health and as an anti-oxidant able to inhibit LDL oxidation. Hence, apoA-I becomes a promising target for CVD treatment. Numerous apoA-I peptides with structural modifications have been designed for therapeutic use. A recently described apoA-I mimetic peptide called FAMP has reported some positive results.
- SOCS proteins is another potential target which is involved in multiple inflammatory pathways. SOCS proteins supress the JAK/STAT pathway, which leads to the production of cytokines and inflammatory factors involved in atherosclerotic processes, including leukocyte recruitment, migration, and proliferation of vascular cells, foam cell formation and apoptosis. SOCS overexpression in mouse models have shown to lower inflammation and cardiovascular disease, driving more research in developing SOCS related peptides for treating CVD.
- Annexin-A1 is a glucocorticoid-regulated anti-inflammatory mediator. It is involved in the activation of the family of formyl peptide receptors that inhibits different processes related to myocardial infarction. Studies have shown that annexin-A1 can directly improve cardiomyocyte viability and contractile function. Moreover, using annexin-A1 peptides have shown cardioprotective effects owing to their anti-inflammatory action that preserves myocardial viability after myocardial infarction.
Current trends and future directions
With great progression in screening methods and bio-computational methods, peptides provide promising tools for precision medicine. The last few years have seen a rise in the number of peptide drug candidates.
One of the emerging trends is the development of multifunctional peptides that can act on more than one target (for example, dual or triple agonists) to increase the pharmaceutical efficacy. Diabetes and cancer has been an active area of research in this context. With respect to type 2 diabetes mellitus, many companies are focusing on developing dual/triple peptides combined with GLP-1. GLP-1–GIP, GLP-1–CCKB, GLP-1–GLP-2 and GLP-1–GCG are some of the drugs in pre-clinical pipelines.
Another major technology that has influenced research in peptide biotherapeutics is the advancement in in-silico methods. Computational PPI docking provides useful structural information at an atomic level that can benefit drug designing. Programs like PepSite 2.0, ClusPro PeptiDock, pepATTRACT, HPEPDOCK and SPRINT-Str are some of the tools used to make accurate predictions on peptide-protein interactions (Lee AL. et al., 2019).
Overall, peptide therapeutics has extended its horizons from hormone replacements to finding more efficient novel molecular targets for various diseases. Integration of technical advancements in biology, chemistry and computation will continue to drive peptide drug discovery. Superior delivery methods and formulations are yet to emerge that can position peptides as one of the most potent class of drugs in the clinic.
Written Shalitha Sasi
(Molecular Biologist and Pharmaceutical R&D Scientist)
References
Joncour V., Laakkonen P. Seek & Destroy, use of targeting peptides for cancer detection and drug delivery. Bioorganic & Medicinal Chemistry, Vol 26, Issue 10,2018. https://doi.org/10.1016/ j.bmc.2017.08.052.
Krenning, E.P.; Bakker, W.H.; Breeman, W.A.; Koper, J.W.; Kooij, P.P.; Ausema, L.; Lameris, J.S.; Reubi, J.C.; Lamberts, S.W. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989, 1, 242–244.
Lau L.J., Dunn M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic & Medicinal Chemistry Volume 26, Issue 10, 1 June 2018, Pages 2700-2707
Lee AL, Harris JL, Khanna KK, Hong J-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. International Journal of Molecular Sciences. 2019; 20(10):2383.
Marqus, S., Pirogova, E. & Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci 24, 21 (2017). https://doi.org/10.1186/s12929-017-0328-x
Morimoto B.H. Enhancing the pharmaceutical properties of peptides. PHARMA HORIZON – vol. 1(2) 2017 Focus on Therapeutic Oligos & Peptides.
Preet P. Peptides: a new therapeutic approach. Int j curr pharm res, vol 10, issue 2, 29-34review article.
Recio C, Maione F, Iqbal AJ, Mascolo N and De Feo V (2017) The Potential Therapeutic Application of Peptides and Peptidomimetics in Cardiovascular Disease. Front. Pharmacol. 7:526. doi: 10.3389/fphar.2016.00526
Sorolla, A., Wang, E., Golden, E. et al. Precision medicine by designer interference peptides: applications in oncology and molecular therapeutics. Oncogene 39, 1167–1184 (2020). https://doi.org/10.1038/s41388-019-1056-3
Thundimadathil J. Cancer Treatment Using Peptides: Current Therapies and Future Prospects. Journal of Amino Acids Volume 2012, Article ID 967347, 13 pages doi:10.1155/2012/967347














