Influence Of HAMA And The Requirement Of SPE In Measurement Of Active GLP-1
Posted by Brandon Savela on Sep 6th 2018
The accurate measurement of active GLP-1 in blood is considered critical for basic and clinical research of diabetes.
Measuring active GLP-1 in blood without the influence of non-specific reactions made by Heterophilic Antibodies (HA), especially HAMA (Human Anti-Mouse Antibodies) is required for obtaining accurate active GLP-1 values and Solid-Phase Extraction: SPE pre-treatment is typically needed. However, there are disadvantages to SPE such as time and additional expenses for the pre-treatment process.
In the following new publication, 63 human plasma samples were collected from 49 volunteers (33: normal glucose tolerance, 8: glucose tolerance, 8: type 2 diabetes). The influence of non-specific reaction by HAMA and requirement of SPE was validated using our assay kit #27784 GLP-1, Active Form and other competitor's kits.
As a result of the study it was suggested that our assay kit #27784 GLP-1, Active Form ELISA is not affected by HAMA and that SPE pre-treatment is NOT required.
Original article for further details: Solid-phase extraction treatment is required for measurement of active glucagon-like peptide-1 by enzyme-linked immunosorbent assay kit affected by heterophilic antibodies. Hasegawa T et al. J Diabetes Investig. 2018 Jul 11. PMID: 29993194
Please refer to the data below. Note that the following data is our in-house data and there is no relation with the study of the new publication.
Reference 1(#27700 IBL without SPE vs #EGLP-35K Millipore SPE)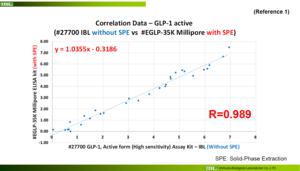
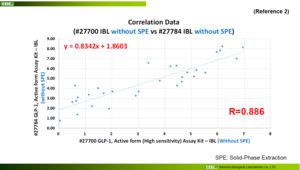
Reference 2 (#27700 IBL without SPE vs #27784 IBL without SPE)
View our comprehensive product offering of incretin related products (GLP-1, GIP and Insulin): Incretin ELISA Kits














