-

Estriol (Free in Saliva)
Competitive immunoenzymatic colorimetric assay for the determination of Estriol (E3) in saliva. For research use only, not for use in diagnostic procedures.
Add to Cart$406.00 -

Estriol (Free in Saliva) 1
Enzyme immunoassay for the determination of free Estriol in saliva. For research use only, not for use in diagnostic procedures.
Add to Cart$256.00 -

Estriol (Free)
Enzyme immunoassay (ELISA) for the quantitative in vitro diagnostic measurement of free estriol (unconjugated estriol) in serum during the second half of pregnancy.
Add to Cart$230.00 -

Estrone (Saliva) 1
Enzyme immunoassay (ELISA) for the determination of Estrone in saliva. For research use only, not for use in diagnostic procedures.
Add to Cart$464.00 -

Estrone ELISA
Enzyme immunoassay (ELISA) for the direct quantitative determination of Estrone in human serum. FDA exempt, can be used for in-vitro diagnostics.<br><br> Estrone is a steroid like estriol and estradiol, belonging to the class of estrogens...
Add to Cart$236.00 -

Estrone RIA
Radioimmunoassay (RIA) for the determination of Estrone in human serum or plasma. FDA registered 510(k) Exempt. For In-Vitro Diagnostic Use.
Choose Options$642.00 -

Estrone-3-Sulfate (Equine)
Enzyme immunoassay (ELISA) for the determination of estrone-3-sulfate in equine serum. For research use only, not for use in diagnostic procedures.
Add to Cart$727.00 -
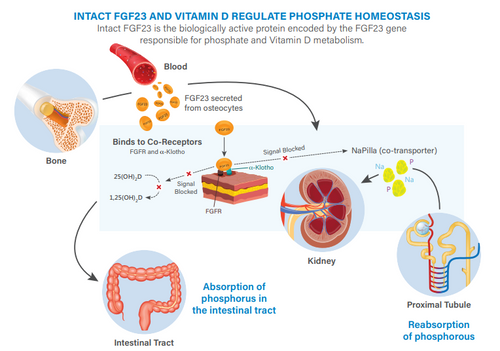
FGF23 (Human Intact FGF23) CLEIA ELISA
FGF23 (Fibroblast Growth Factor 23) is a protein belonging to the fibroblast growth factor family. FGF23 is involved in the regulation of phosphorus metabolism. FGF23 has a molecular weight of approximately 32 kDa. FGF23 is produced in bone cells. In...
Add to Cart$1,382.00 -

Follicle Stimulating Hormone (FSH) ELISA
Solid phase enzyme immunoassay (ELISA) for the determination of FSH in human serum. For research use only, not for use in diagnostic procedures.
Add to Cart$203.00 -

Free T3 RIA
Radioimmunoassay (RIA) for the determination of human free triiodothyronine (T3) in serum or EDTA plasma. For research use only, not for use in diagnostic procedures.
Choose Options$341.00 -

Free T4 RIA
Radioimmunoassay (RIA) for the determination of human free thyroxine (T4) in serum or EDTA plasma. For research use only, not for use in diagnostic procedures.
Choose Options$320.00 -

Free Thyroxine (T4) ELISA
Solid phase competitive enzyme immunoassay (ELISA) for the determination of Free Thyroxine (fT4) in human serum. For research use only, not for use in diagnostic procedures.
Add to Cart$203.00

Endocrinology & Hormones
Endocrinology is the study of medicine that relates to the endocrine system which is the system that controls hormones.
This category lists test kits using ELISA, RIA / IRMA, and luminescence technologies for human and various animal testing in blood, urine and salivary samples.
Saliva testing mainly includes the measurement of free steroid hormones.
